HOME
案例實績
Chemical & Pharma
Why remove endotoxins?
2025-11-10
Why remove endotoxins?
Recently, while visiting pharmaceutical clients, I've frequently heard the keyword "[endotoxin]".
But what is endotoxin?
Under what circumstances is it produced?
The image below a common Gram-negative bacteria.

Bacteria can be broadly classified into two categories: Gram-positive bacteria and Gram-negative bacteria.Endotoxins primarily originate from the outer membrane of the cell wall of Gram-negative bacteria.
This is the outermost protective layer of the bacteria in the image, which we call the cell wall.
The cell wall, like human skin, primarily serves a protective function.
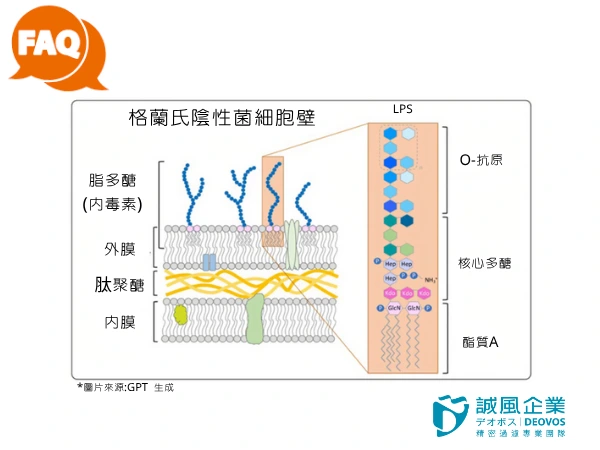
If you examine the cell wall surface under magnification, you can refer to the image for its structure.
When these bacteria grow, lyse, die, or their cell walls detach naturally, endotoxins are released into the environment (such as water, culture medium, and pharmaceutical solutions).
The core component of endotoxin is lipopolysaccharide (LPS),
which has a structure consisting of three parts:
Lipid A
Core polysaccharide
O-antigen
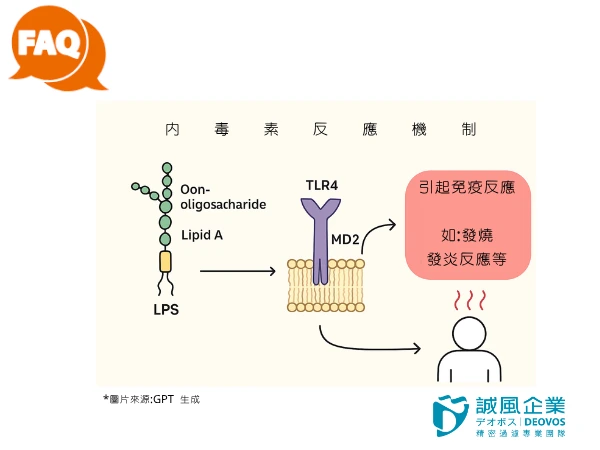
As mentioned earlier, when bacteria lyse or die, their cell walls detach, releasing endotoxins.
The toxicity of endotoxins (LPS) primarily comes from Lipid A, as they strongly activate the human immune system, triggering reactions such as fever, sepsis, and shock.
Because endotoxins cause an immune response, they are a primary concern in the biomedical, pharmaceutical, and cell culture fields.
We will share information on how to remove or prevent endotoxin contamination in products in the next article.
DEOVOS often says, "There's no such thing as the 'best' filter; there's only the 'most suitable' filter."
Our team can provide the most appropriate solution based on your specific product requirements or budget.
So, how do you choose the right air filter for your factory?
Contact DEOVOS professional filtration experts to discuss your specific needs.
